Inside the procedure at the heart of a multibillion-dollar race to a safer AFib treatment
Published in Business News
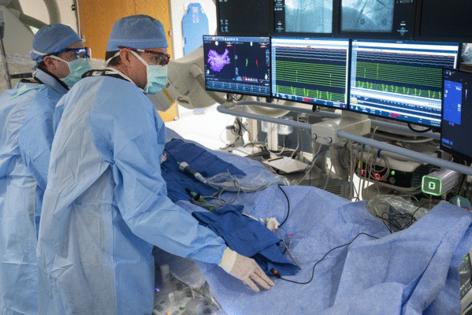
Under a canopy of eight heart-monitoring screens in a cardiac catheterization lab on a recent Monday, Mayo Clinic doctors briefly paused a procedure treating atrial fibrillation to inspect a new tool: a long tube with what looked like flower petals made out of wire at its end.
That wiry flower is the focus of a multibillion-dollar innovation race between some of the world’s largest medical technology companies.
Medtronic and Boston Scientific, which both have major cardiac-device operations in Minnesota, and Johnson & Johnson MedTech are vying to control the large and fast-growing market for minimally invasive medical devices to treat the widespread heart problem atrial fibrillation with a safer and quicker procedure called pulsed field ablation (PFA).
PFA uses electric pulses instead of extreme heat or cooling to ablate cardiac tissue around pulmonary veins on the heart, blocking bioelectric signals in the tissue that cause the heart’s atrial chambers to quiver, or fibrillate. Atrial fibrillation, or AFib, is believed to affect more than 10 million Americans, greatly increasing their risk of having a stroke, the latest scientific estimates show.
Doctors say the new ablation procedure cuts down a patient’s time in the cath lab by hours, and reduces risks for serious complications compared to older techniques. Medtech executives expect the technology to help fuel their companies’ future growth.
Boston Scientific CEO Mike Mahoney has said the company’s new Farapulse ablation system is “the most transformational product that I’ve seen in my career.” Medtronic CEO Geoff Martha has said, “We’re at one of those moments in medtech where a new technology is causing a rapid shift in the treatment of a disease. In this case, PFA is that technology.”
Analytics company Clarivate projects pulsed field ablation devices will surpass $1.3 billion in sales globally this year, and the total number of procedures performed will at least quadruple in the next two to three years as PFA makes up an increasingly large share of all ablations performed.
The Farapulse system was first to market in Europe, gaining regulatory approval in early 2021, before Boston Scientific acquired it later that year. The U.S. Food and Drug Administration then approved Medtronic’s system, called PulseSelect, last December. Then Farapulse, which includes hardware built in Minnesota, received FDA approval in January. Johnson & Johnson MedTech also received FDA approval for its Varipulse system earlier this month.
The companies are now pushing to commercialize premium PFA systems that map the heart and reduce incisions into the body as the technology floods hospitals.
Tony Crisci of Ocean City, N.J., said he held off for a procedure at Mount Sinai in New York until the new technology became available.
“I thought I was just getting older, and I couldn’t work out as hard [without getting] out of breath,” Crisci, 67, said of his condition before he received treatment with Boston Scientific’s system. “But now I feel like 20 years younger.”
In the Mayo Clinic heart-catheterization lab, a patient was on the table swarmed by health care professionals, wires and massive devices that made the room look like the inside of a spacecraft by roughly 8:30 one recent morning. The ablation wrapped up by 10:30 a.m.
That’s not how quickly AFib procedures formerly went.
Older radiofrequency ablation tools use heat, taking minutes for each round of ablation near the pulmonary vein. During PFA, each electric pulse is delivered in a matter of nanoseconds during a secondslong delivery window, reducing the procedure’s time by hours. Mapping and monitoring the heart seemed to take longer than the ablation during the Mayo Clinic procedure.
AFib is an irregular heart rhythm when the heart’s upper chambers chaotically beat out of sync with the lower chambers, increasing risk of stroke and heart failure. Mayo Electrophysiology laboratory director Dr. Suraj Kapa said incidence of the arrhythmia is expected to increase as the population becomes older and the world faces an obesity epidemic.
Common symptoms include a racing or fluttering heart, lightheadedness, chest pain and extreme fatigue, but some patients have no symptoms: “It can range from somebody being totally asymptomatic — they don’t feel it at all — all the way to somebody feeling essentially like a truck ran them over,” Kapa said.
Crisci, who had a PFA procedure at Mount Sinai, said he felt short of breath whenever he exerted himself in recent years. A doctor discovered his AFib during a routine physical in 2022.
Patients can control AFib through medications or ablation procedures, but there’s no true cure.
Dr. Khaldoun Tarakji, chief medical officer of Medtronic’s cardiac ablation unit, said anti-arrhythmic drugs can cause potentially fatal side effects. Boston Scientific Chief Medical Officer Dr. Ken Stein said drugs are often ineffective.
“Even a decade ago,” Stein said, “it was clear that some type of ablation procedure was ultimately going to be preferred versus drugs for patients.”
Pulsed field ablation is at least as effective and is certainly more efficient than conventional thermal ablation, which includes radiofrequency and cryoablation methods, Stein said.
During a cryoablation procedure, a balloon expands inside a pulmonary vein, freezing tissue with extreme cold, said Dr. Henri Roukoz, director of electrophysiology at the University of Minnesota Medical Center.
Radiofrequency ablation, which is more common, cauterizes tissue around the pulmonary vein point by point, in intervals that take minutes and add up to hours. Using thermal sources, doctors can inadvertently damage other organs like the esophagus, leading to rare but potentially fatal complications, Roukoz said.
Pulsed field ablation uses a high voltage impulse to create pores in cells in the area surrounding the pulmonary vein, causing them to disintegrate and die, doctors said. Because the pulses are localized to targeted cells, doctors said there’s a smaller chance the procedure affects adjacent organs.
Crisci said physicians offered him drugs or ablation but he declined long-term medication for treatment: “I don’t take aspirin or anything.” He said he held off on the procedure until he landed a spot in Johnson & Johnson MedTech’s clinical trial.
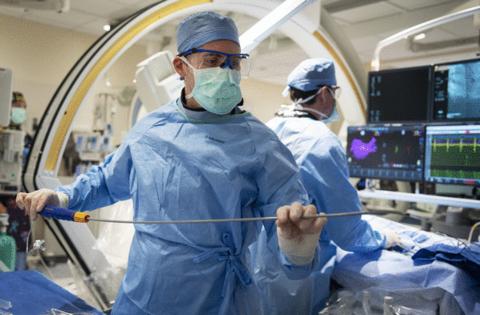
In the Mayo Clinic cath lab, Dr. Peter Noseworthy and Dr. Robert Ward made two incisions on the left and right side of the patient’s groin to insert catheters, which are thin tube-shaped devices physicians can advance to the heart through blood vessels, allowing access to the heart without open surgery.
The doctors threaded a mapping catheter — with several tiny legs sprouting from its end — through the groin, moving it up blood vessels until it reached the heart. There, its metallic legs danced across the interior surface of the heart as the physician toggled the device’s controller. This produced a model of the heart that allowed the physicians to precisely identify spots for ablation.
Then the physicians threaded a Farapulse catheter into the right incision and snaked it toward the heart. They poked a hole in the heart’s interior so the catheter could move from the right upper chamber to the left upper chamber, which the arrhythmia affects.
The doctors delivered roughly eight electric pulses to the heart. Each pulse gets delivered after the tap of an iPad-like touchscreen. The procedure wraps up quickly.
It’s at least as effective as radiofrequency ablation, but the arrhythmia can return, doctors say.
Crisci said he healed quickly following his first ablation in March using the J & J technology, but he went back into AFib about a week and a half later. He received a second procedure in July, and he said he hasn’t had AFib since. Now, he’s hitting the gym more.
“It’s really giving me a second youth,” Crisci said.
At the electrophysiology divisions of the biggest medtech companies, executives spotted the promising market years ago. Boston Scientific acquired University of Iowa startup Farapulse in 2021 for hundreds of millions of dollars after first investing in the company in 2014. In 2022, Medtronic acquired Affera, the company behind its new catheter that ablates and maps the heart, for $925 million.
Stein said physicians use Farapulse in the large majority of pulsed field procedures. Its Minnesota-based division within Boston Scientific grew by 177% on an organic basis during the most recent quarter.
Jasmina Brooks, president of Johnson & Johnson MedTech’s electrophysiology division, said the company’s pulsed field system was the first in the U.S. to fully integrate with the company’s 3D heart-mapping system, which helps doctors visualize the heart as they position the catheter and deliver the energy.
And Tarakji of Medtronic said the adoption of PFA “has even exceeded the wildest expectation.”
With PulseSelect, he said patients often don’t experience some of the side effects of radiofrequency ablation like chest pain. He pointed out that that the company’s catheter has one of the smallest diameters, which can reduce recovery time.
The race doesn’t come without obstacles. A supplier problem held back Medtronic PulseSelect sales for the most recent reported quarter. Boston Scientific paused an important clinical trial to expand the use of its technology as a first-line treatment for persistent AFib after making unanticipated observations — and then restarted it weeks later.
The companies are now incorporating mapping technology into the ablating catheter to further streamline the procedure. And Tarakji said Medtronic is in early feasibility studies to study whether the company’s technology can treat a serious arrhythmia called ventricular tachycardia.
Nick Spadea-Anello, president of Boston Scientific’s electrophysiology division, said the the new technology will fuel future innovation.
Said Stein: “The potential now is to offer them ... a therapy that is safe, a therapy that’s effective and a therapy that is efficient for the system as a whole: It just changes patients’ lives.”
©2024 The Minnesota Star Tribune. Visit at startribune.com. Distributed by Tribune Content Agency, LLC.













Comments