Current News
/ArcaMax

The Camden Diocese will no longer oppose a statewide investigation of clergy sex abuse, new bishop says
The new bishop of Camden said Monday that his diocese would no longer oppose a statewide investigation of sex abuse by clergy — a stunning reversal after the diocese had spent years arguing in sealed court documents that the probe proposed by the New Jersey Attorney General's Office should not be allowed to move forward.
In an interview, ...Read more

Jury selection begins in Diddy's sex trafficking trial in NYC. What we know
Jury selection for the federal criminal trial of Sean “Diddy” Combs began on Monday morning in New York. The high-profile defendant has been behind bars for eight months awaiting trial for charges including racketeering, transportation to engage in prostitution, sex trafficking, as well as an additional charge of forced labor against ...Read more

Santa Clara County, SF join lawsuit against Trump's 'unlawful' conditions on homelessness funds
Santa Clara County and San Francisco, along with seven other local governments, are asking a federal judge to block the U.S. Department of Housing and Urban Development from placing “unlawful” conditions on federal funds — stipulations that could put millions of dollars to address homelessness at risk.
The lawsuit, which is being led by ...Read more
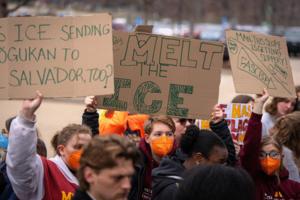
Judge says Minnesota student can't be deported, but Department of Homeland Security files appeal
An immigration judge has terminated the deportation case against Doğukan Günaydin, the University of Minnesota student who was arrested in late March, although he is expected to remain in custody as the federal government appeals the decision.
Immigration Judge Sarah Mazzie filed the ruling on Thursday. It came days after U.S. federal court ...Read more

Trump blocks Harvard from new research funding in latest blow
The Trump administration is declaring Harvard University ineligible for new research grants from the federal government in the latest escalation between the White House and the Ivy League school.
Education Secretary Linda McMahon sent the university a letter warning that access to additional federal funding would not be possible until “they ...Read more
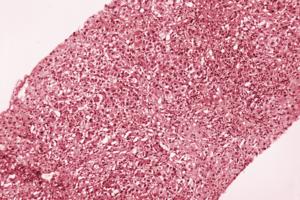
Hepatitis A outbreak declared in L.A. County. 'We really have to get ahead of this'
Los Angeles County has declared a communitywide outbreak of hepatitis A, a highly contagious viral disease that can lead to lasting liver damage or even death.
Although cases of hepatitis A are nothing new in the region, health officials are now expressing alarm both at the prevalence of the disease and who is becoming infected.
The total of ...Read more

ks // 'We have to stop': Atlanta Council urges state patrol to curb high-speed chases
ATLANTA — The Atlanta City Council on Monday took its most forceful steps yet to try to curb high-speed pursuits involving the Georgia State Patrol, weeks after a chase led to a crash that killed a 19-year-old bystander in Little Five Points.
The council unanimously approved a resolution urging the state to amend GSP’s pursuit policy to ...Read more

Mass. Gov. Maura Healey, five other governors invite Canadian premiers to Boston to talk Trump tariffs
Massachusetts Gov. Maura Healey and five other northeastern governors invited Canadian premiers to Boston to discuss the impacts of President Donald Trump’s tariffs and how the two countries can maintain “strong trade relations that benefit local businesses and residents,” Healey’s office said Monday.
The potential future gathering, ...Read more

Here's what the Trump administration proposes spending on Washington's toxic Hanford nuclear site
TACOMA, Wash. — The Trump administration showed support for environmental cleanup at Hanford in its initial proposal for fiscal 2026 funding, calling for a much higher annual budget than it did during Trump’s previous term as president.
The support for Hanford came as elsewhere in the proposed budget the Trump administration wants a $163 ...Read more

Leaked contract shows how Cuba pockets money Bahamas pays for medical services
The Bahamian government appears to have signed off on a contract that directs most of the money paid for the services of four Cuban health professionals to a Cuban government entity while giving away its legal authority on key issues to the communist-ruled island, according to a copy obtained by a group monitoring Havana’s medical missions ...Read more

N.J. Attorney General wants Bob Menendez barred from office for life
The New Jersey Attorney General's Office asked a state judge on Monday to declare that former U.S. Sen. Bob Menendez is barred for life from seeking public office following his bribery conviction.
Menendez was sentenced in January to 11 years in prison for taking illegal gifts and advancing the Egyptian government's interests. Following his ...Read more
News briefs
Trump directs Bureau of Prisons to rebuild and reopen Alcatraz. Can he do that?
President Donald Trump said Sunday that he was ordering the FBI to reopen the Alcatraz Federal Penitentiary, the historic prison on an island off of San Francisco that has been closed since 1963.
“REBUILD, AND OPEN ALCATRAZ!” he wrote in a post on Truth Social....Read more

'It's in a chaotic state': Virginia food banks feel the effects of federal funding cuts
Bob Latvis knows there may often be changes in federal funding for food banks with any new administration. This time though, many Virginia food banks are in a state of limbo as they wait for federal support.
The first Trump administration provided trade mitigations that increased available food for food banks, and the Biden administration ...Read more

Hegseth says 'lean and mean' military requires cutting officers
WASHINGTON — Defense Secretary Pete Hegseth ordered a reduction of the U.S. military’s highest-ranking officers — potentially setting up a clash with Congress, which must approve any such adjustments.
In a memo released Monday, Hegseth called for slashing 20% of four-star positions in the active-duty military, 20% of all general officers ...Read more

Alberta's Smith plans talks with Carney, says separation vote possible
TORONTO — Alberta Premier Danielle Smith said she’s appointing a group to negotiate with the Canadian government on removing laws that restrict energy production, adding that a referendum on the province separating from Canada may be on the ballot as soon as next year.
Alberta will seek an agreement that guarantees energy transportation ...Read more

No new trial for white supremacist convicted in plot to disrupt Baltimore power grid
BALTIMORE — A federal judge rejected a white supremacist’s request to be tried again after the Florida resident was convicted of a conspiracy charge stemming from a plot to disrupt the Baltimore-area power grid.
Lawyers for Brandon Clint Russell, 29, had filed the motion for a new trial after the Department of Justice revealed additional ...Read more

Nearly quarter of people on long-acting opioids develop addiction
More than one in five people prescribed extended-release painkillers such as OxyContin developed an addiction within a year, according to a newly released study mandated by the U.S. Food and Drug Administration.
The study, repeatedly delayed by more than a decade and released Monday, revealed a far higher percentage of pain patients addicted ...Read more

Federal judge orders North Carolina to certify Riggs as winner in Supreme Court election
RALEIGH, N.C. — In a ruling that could put an end to nearly six months of legal battles over North Carolina’s contested Supreme Court election, a federal judge on Monday ruled against the Republican candidate’s effort to overturn his narrow loss.
Chief U.S. District Judge Richard E. Myers, an appointee of President Donald Trump, ruled ...Read more

New York Times Leads Pulitzers With 4
NEW YORK — The New York Times won four Pulitzer Prizes, including the Explanatory Reporting award for its examination of the failures and missteps made by the United States in Afghanistan.
Columbia University’s Graduate School of Journalism announced the 109th annual Pulitzer Prizes Monday in New York. The awards honored the best reporting ...Read more

EPA tells hundreds of Research Triangle Park scientists to reapply for jobs under Trump office cut
The Environmental Protection Agency is reorganizing its science research office in a move expected to impact, and likely eliminate, several hundred jobs at the agency’s Research Triangle Park campus in North Carolina.
During a virtual meeting Friday, EPA leaders encouraged staff within the Office of Research and Development to reapply for ...Read more
Popular Stories
- AG Dana Nessel drops charges against group arrested in University of Michigan pro-Palestinian camps
- US Rep. Jan Schakowsky, Illinois congresswoman since 1999, announces she will not seek another term next year
- Trump administration offers $1,000 to immigrants who return home voluntarily
- Venezuelan opposition leader Edmundo González hospitalized in Spain
- Trump administration offers undocumented immigrants $1,000 to leave the country





